Clinical Real World Evidence and Medical Research
enabled through 5000+ centers and 1bn+ clinical parameters

partner centers
with data and site
partnerships

partner centers
with data and site
partnerships

unique patient lives
integrated and
managed

unique patient lives
integrated and
managed

years longitudinal
data and patient
journeys across
therapy areas

years longitudinal
data and patient
journeys across
therapy areas

therapy areas and
disease conditions
(acute, chronic,
super-specialty and rare diseases)
therapy areas and
disease conditions
(acute, chronic,
super-specialty and rare diseases)

publications and
white papers
across medical
journals and
conferences

publications and
white papers
across medical
journals and
conferences


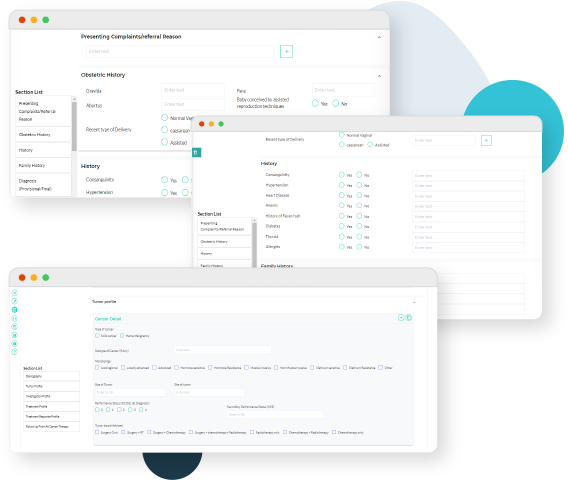
Hypothesis building and testing to structure effective end points and outcomes
Best-in-class clinical operations, biostatistics, and medical writing teams and processes with 20+ SOPs in-line with the regulatory guidelines and approved by global pharma companies
Collaboration with hospitals, diagnostic labs, practitioners, and independent ethics committees
Identified and Prioritized journals to ensure publication and visibility across right platforms
eCRF to enable digital data compilation in the
new-age world
Extensive Quality Control backend engine and
workflows to ensure continued data monitoring
and site follow-ups
Detailed Tech and IT SOPs in compliance with the
data, confidentiality, security guidelines
20+ SOPs spanning each micro-step of clinical operations, evidence generation and medical research
SOPs led management of day to day activities of retrospective and prospective studies
Detailed technology and IT SOPs to ensure compliance with local guidelines
Retrospective real world data based clinical studies,
enabled by proprietary clinical data collaborations and
provider partnerships
Observational studies and long-term Registry
programs across a network of 5000+ sites
Phase 4 Clinical Operations, Prospective, and Post
marketing surveillance studies
HEOR hypothesis, modelling and studies


Proprietary data and evidence backed scientific content and medical communications
Digital-ready and digital-effective scientific communications to enable new-age product commercialization
Trackable micro-sites to enable seamless monitoring of customer interactions and preferences w.r.t. scientific evidence
Real-time Epidemiology platforms
Infectious Disease Alerts based Surveillance
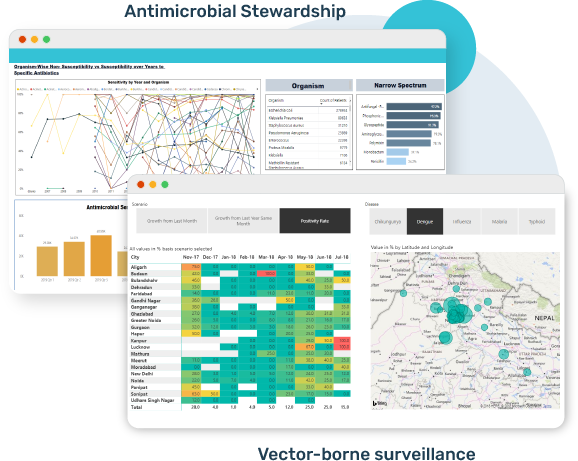
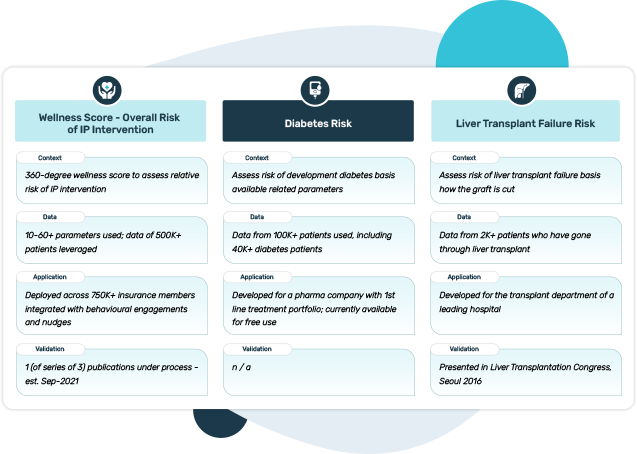
Risk Assessment – Outpatient and Inpatient
Disease specific risk algorithms
Empirical Treatment Support Platforms
Intervention / Surgical Decision Support